A Validated LMS for Clinical Trial Training and Documentation
Transform your clinical trial training with a validated LMS designed specifically for Clinical Study Companies and CROs. Ensure compliance, maintain data integrity, and streamline the training process for sponsors, investigators, and study teams.
Trusted by Leading Clinical Research Organizations








Why Do CROs Choose Dokeos for their Validated LMS?
Dokeos LMS addresses the specific challenges faced by Clinical Research Organizations. Our platform combines regulatory compliance, operational efficiency, and comprehensive validation to support your clinical trial success.

Built-In Regulatory Compliance
- FDA 21 CFR Part 11 compliant
- Electronic signatures and audit trails
- Complete training documentation for regulatory inspections
- HIPAA and patient data privacy compliance

Clinical Trial-Readiness
- Training tracking for multi-site studies
- Automated Trial Master File documentation
- Investigator and study team qualification management
- Integration with Clinical Trial Management Systems
Comprehensive Validation Package
- Full Computer System Validation (CSV) documentation
- Installation, Operational, Performance Qualification (IQ/OQ/PQ)
- Validation maintenance and change control
- Regular system updates with re-validation support
Ensuring Clinical Trial Accuracy with Dokeos LMS
In the complex world of clinical research, maintaining training accuracy is crucial for study success. Learn how our LMS streamlines the entire training process while ensuring complete compliance and documentation.

Study-specific training deployment

Multi-site investigator training

Protocol amendment training tracking

Sponsor oversight capabilities

Real-time compliance monitoring

Integration with clinical systems
Advanced Features for Clinical Research
Our platform includes specialized features specifically designed for clinical research operations. Each component is built to support regulatory compliance while maximizing operational efficiency.
- Certificate Management
- Electronic Signature System
- Module Presence & Completion Tracking
- Advanced Quality Control
- Audit-Ready Reporting
- Trial Master File Integration
- Investigator Qualification Management
- Multi-Sponsor Support
- Protocol Training
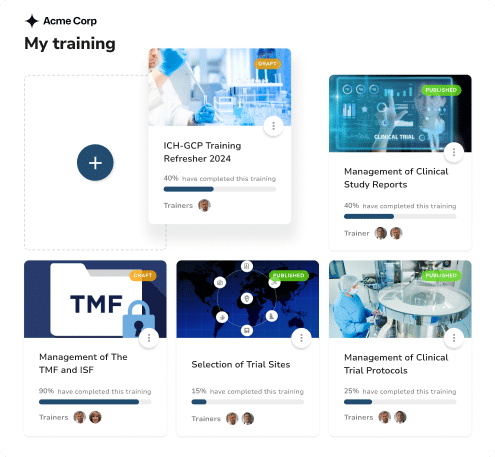
- Centralized dashboard for tracking all study-related certifications
- Automated expiration notifications and renewal tracking
- Timestamped certificate generation for regulatory compliance
- Training certificate verification for investigators and study staff
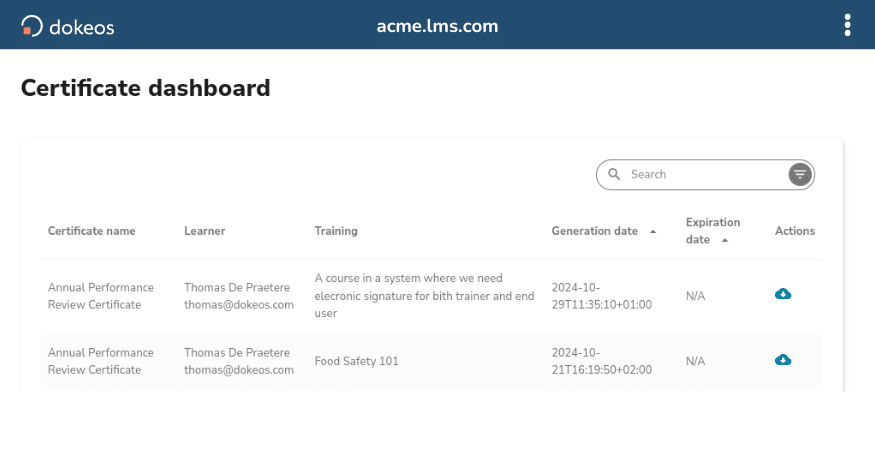
- 21 CFR Part 11 compliant electronic signatures
- Two-factor authentication for secure identity verification
- ‘Read and Understood’ tracking
- Complete signature audit trail for regulatory inspection
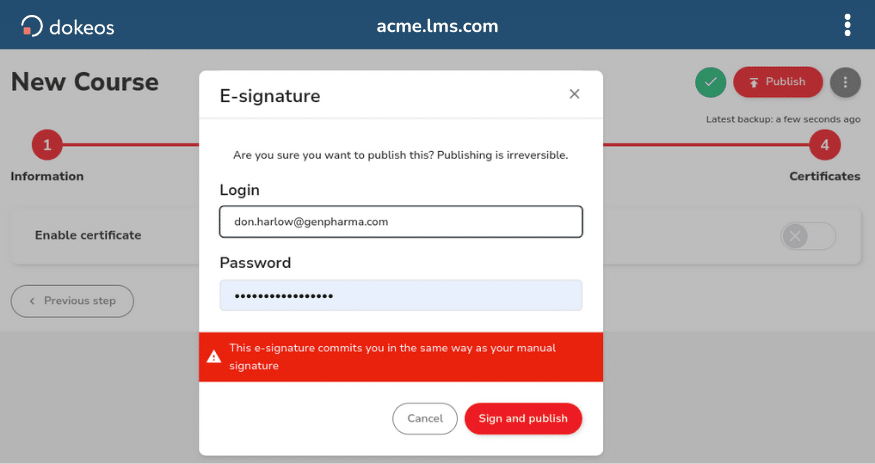
- Real-time monitoring of training module participation
- Automated attendance tracking for virtual sessions
- Detailed time tracking and engagement metrics
- Protocol training completion verification
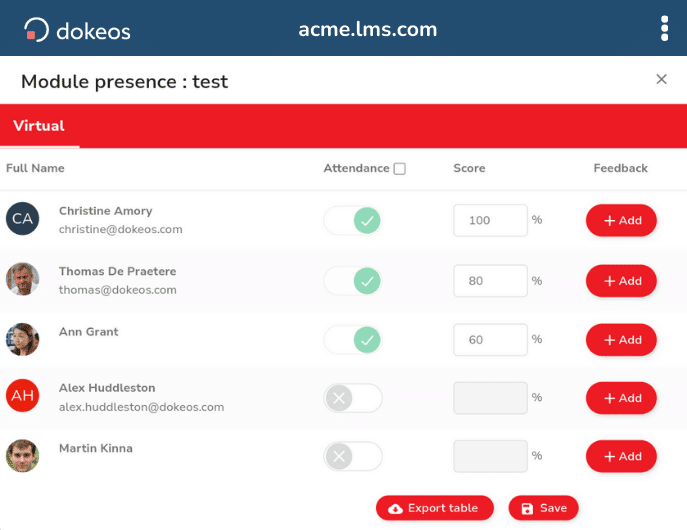
- Built-in quiz validation tools
- Question analysis and performance metrics
- Training effectiveness assessment
- Automated quality assurance reporting
- Custom report generation for sponsor oversight
- Real-time compliance monitoring dashboards
- Training gap analysis tools
- Automated audit trail generation
- Detailed timestamp tracking for all system activities
- Seamless TMF documentation export
- Automated training record filing
- Version control and document tracking
- Regulatory inspection-ready documentation
- Centralized investigator credentials tracking
- Automated qualification expiration alerts
- CV and training certificate repository
- Site-specific qualification requirements
- Secure data segregation between sponsors
- Customized training programs by sponsor
- Sponsor-specific reporting capabilities
- Role-based access control
- Protocol amendment version control
- Training completion tracking
- Protocol-specific assessment tools
- Impact analysis for protocol changes
Discover 80+ Certified Courses for CROs
Access validated, off-the-shelf training content designed specifically for clinical research professionals. Our GxP Training Catalog helps ensure consistent quality across all study sites while saving valuable time and resources.
Partner with our Clinical Trial experts
Ready to transform your clinical trial training management? Contact our team to learn how Dokeos LMS can support your CRO’s specific needs.
Dr. Patrick Jonvel
40 years in pharma with ALCON, ELI LILLY, MERCK & IPSEN. Expert in quality, compliance, and training, with 15+ FDA inspections and no major findings. Created 100+ courses and delivered 40K+ hours of GLP/GMP training.
Dr. Thomas De Praetere
Founder of Dokeos, has 20+ years of experience in learning technologies and regulated industries, specializing in pharma and medical devices. As a consultant Quality Manager at Dokeos, he ensures GxP and FDA compliance for training systems in the life sciences sector.
Christine Amory
Christine Amory, Chief Customer Officer at Dokeos, has 15+ years of experience in e-learning and regulatory compliance for pharma and healthcare. She specializes in developing tailored training programs and ensuring adherence to standards like HIPAA and GDPR.
Stephan Atsou
Stephan Atsou, CEO of Dokeos, brings 25 years of expertise in digital learning and corporate training. Formerly leading CrossKnowledge Benelux, he specializes in aligning learning strategies with business goals and is co-author of a guide on e-learning for companies.
Frequently Asked Questions
Dokeos offers certified training modules, automated reporting, and detailed recordkeeping aligned with ISO 13485 standards. Our platform ensures your team is always prepared for audits and regulatory requirements.
Yes, Dokeos LMS supports multi-site training management with role-based access control, automated notifications, and centralized reporting.
When protocols are updated, the system automatically tracks version control, notifies affected team members, and documents completion of amendment training.
Yes, Dokeos LMS comes with full Computer System Validation documentation and maintains validation through our controlled change management process.

Still have questions?
Fill out this form to contact us. A compliance expert will get back to you shortly.
Trusted by Leading Clinical Research Organizations



























