Empowering Medical Device Organizations with an FDA-Compliant LMS
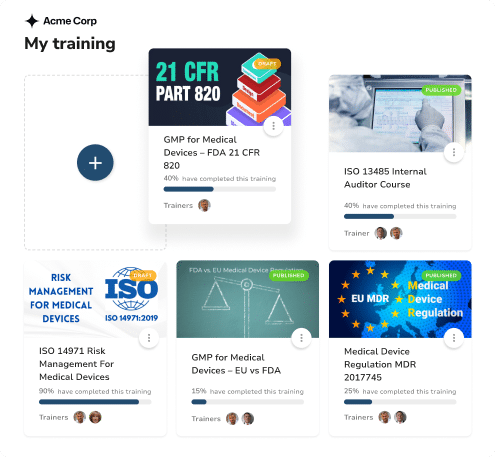
Optimize time-to-compliance, just like our clients






Meet today’s regulatory requirements, adapt for tomorrow’s
Dokeos LMS delivers a flexible, traceable compliance solution to ensure you’re always audit-ready.
Meet regulatory standards anytime
Dokeos LMS delivers a flexible, traceable compliance solution to ensure you’re always audit-ready.

Empower teams with traceable, verified training
Dokeos provides verified training certifications, ensuring every course completion is adequatly documented.

Simplify documentation and reporting
Maintain a clear audit trail and automated reports for a seamless inspection process.
FDA ISO 13485 compliance made simple for Medical Devices Organizations
Ensure your medical devices are inspection-ready with a tailored LMS designed to meet FDA ISO 13485 standards. Dokeos LMS helps you handle the complexities of software development, documentation, and regulatory requirements — all in one place.

Device Compliance
Demonstrate your quality system’s ability to identify and resolve deficiencies effectively.
Code Compliance
Ensure full traceability with ALCOA+, versioning, and GIT workflows.
Data Compliance
Ensure your medical data and databases meet ISO requirements.

Clinical Trials
Demonstrate your quality system’s ability to identify and resolve deficiencies effectively.
Training Reporting
Manage logs, certificates, and training files with an integrated system.
QMS
Ensure the compliant link between your LMS and your QMS. We help you select the best QMS for you.
Dokeos brings everything you need for compliance
in a validated and modern interface LMS
Dokeos LMS provides features, documentation and processes to meet regulatory requirements in the Medical Devices Industry. We succeed in being the best combination of a Validated Training System with a Modern User Experience.
- Validated LMS
- Video Conferencing
- Audit Trail
- Audit Readiness
- Individual Training File
- Electronic Signature
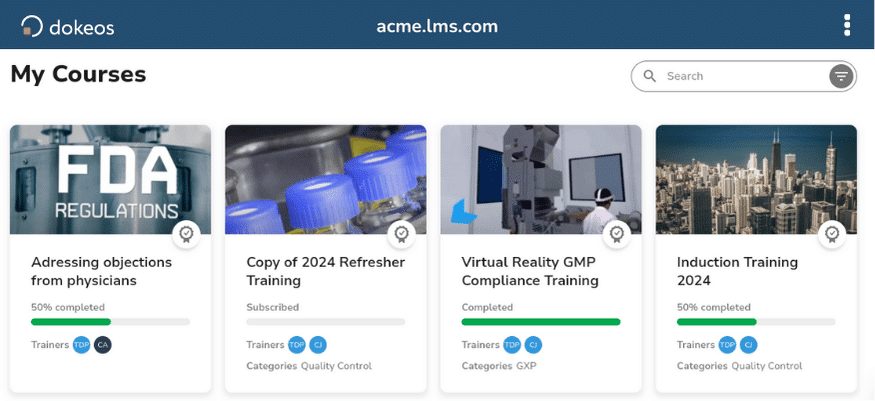
A compliant e-learning platform with a user-friendly interface.
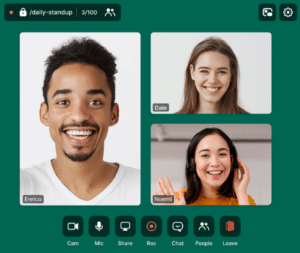
Integrate virtual training sessions within the LMS to track attendance.
Complete and searchable system logs for all events required during an inspection.
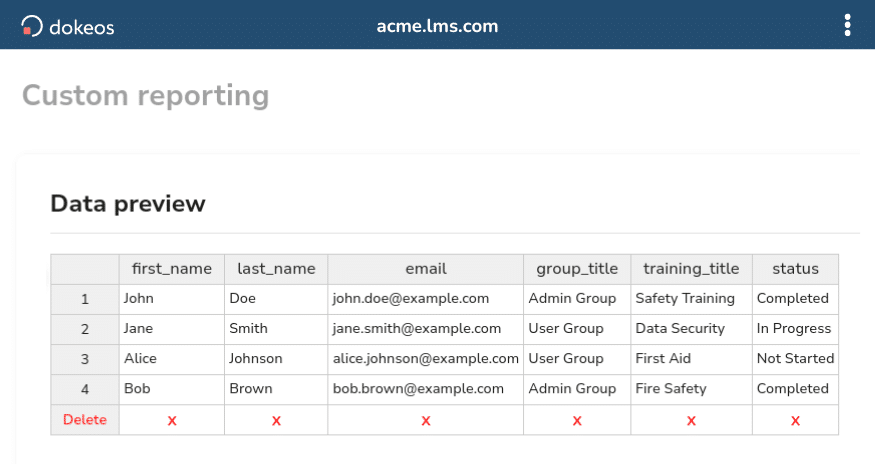
Our team of experts help you prepare more than 15 different audits.
Generate timestamped and authenticated audit-ready user reports.
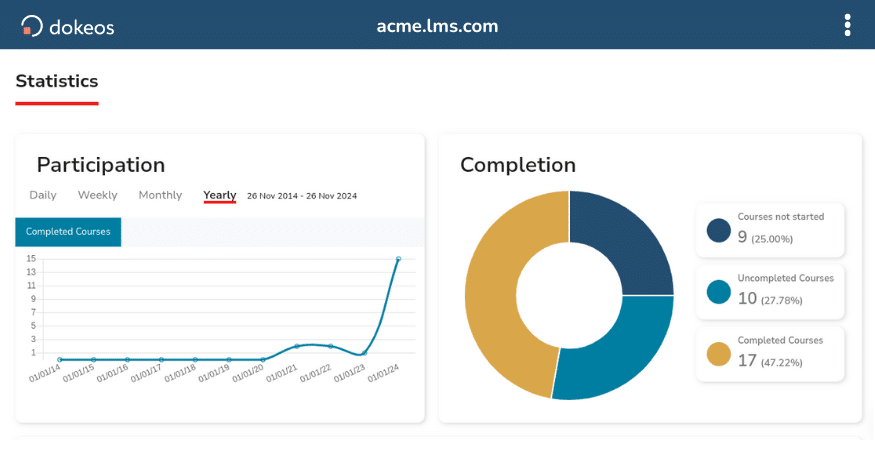

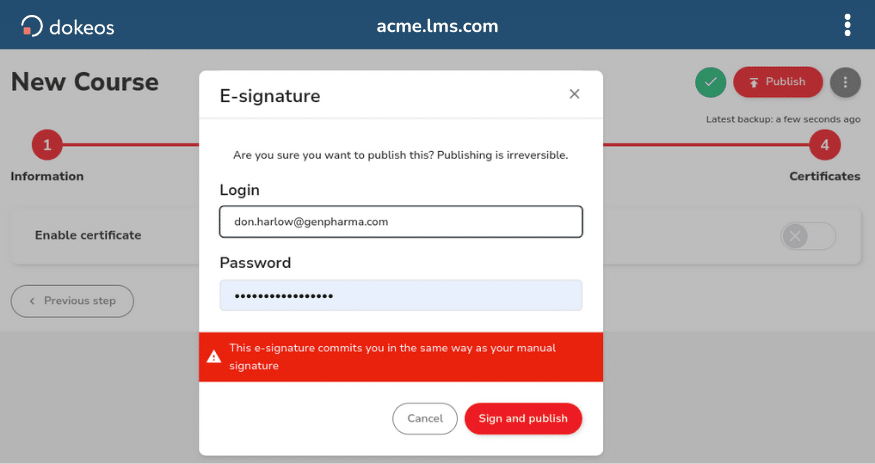
Authors, reviewers, and employees use Electronic Signatures to validate content and course completion.
Discover 80+ Certified Courses for Medical Device Professionals
You can integrate a certified course catalog (80+ trainings) directly into your Dokeos platform, delivering a complete, ready-to-use learning solution from day one. Upon course completion, your team will earn FDA-compliant certificates along with CPD/CEU credits.






Compliance for any Medical Devices Organizations, large or small

For Compliance Teams
Keep documentation audit-ready with ease using Dokeos’ flexible tools.

For HR Managers
Simplify onboarding with pre-structured, compliant training courses.
For Training Coordinators
Manage refresher courses, training files, and certifications in one platform.
Partner with our compliance experts
Dokeos’ experts deliver tailored strategies to ensure regulatory alignment and audit readiness.
Dr. Patrick Jonvel
40 years in pharma with ALCON, ELI LILLY, MERCK & IPSEN. Expert in quality, compliance, and training, with 15+ FDA inspections and no major findings. Created 100+ courses and delivered 40K+ hours of GLP/GMP training.
Dr. Thomas De Praetere
Founder of Dokeos, has 20+ years of experience in learning technologies and regulated industries, specializing in pharma and medical devices. As a consultant Quality Manager at Dokeos, he ensures GxP and FDA compliance for training systems in the life sciences sector.
Christine Amory
Christine Amory, Chief Customer Officer at Dokeos, has 15+ years of experience in e-learning and regulatory compliance for pharma and healthcare. She specializes in developing tailored training programs and ensuring adherence to standards like HIPAA and GDPR.
Stephan Atsou
Stephan Atsou, CEO of Dokeos, brings 25 years of expertise in digital learning and corporate training. Formerly leading CrossKnowledge Benelux, he specializes in aligning learning strategies with business goals and is co-author of a guide on e-learning for companies.
Frequently Asked Questions
Dokeos offers certified training modules, automated reporting, and detailed recordkeeping aligned with ISO 13485 standards. Our platform ensures your team is always prepared for audits and regulatory requirements.
Yes, Dokeos provides training and documentation solutions specifically designed for FDA 21 CFR PART 820. From creating courses to maintaining secure audit trails, we simplify compliance for medical device companies.
Our LMS provides secure documentation storage, automated audit trails, and real-time reporting tools, making it easy to organize and present records during inspections.
Absolutely. Dokeos integrates seamlessly with your QMS, creating a unified compliance ecosystem that supports training, documentation, and reporting.
Yes, Dokeos offers powerful course design tools, enabling you to create and customize training tailored to ISO, FDA, or company-specific protocols. This ensures compliance and minimizes risk.
Dokeos automatically tracks training progress, completion, and certification with secure e-signature verification. This ensures that all records are audit-ready and compliant with regulatory standards.
You can start with a 14-day free trial to explore the platform’s capabilities or request a 30-minute demo to see how Dokeos can meet your organization’s specific compliance needs.

Still have questions?
Fill out this form to contact us. A compliance expert will get back to you shortly.
Optimize time-to-compliance, just like our clients