Since its introduction in 1997, the FDA’s 21 CFR Part 11 has provided rules on the management of electronic records and electronic signatures in the pharmaceutical and biotechnology industries. The latest update was published on October 1, 2024. It provides a guide to using electronic records in clinical investigations, helping stakeholders adhere to good clinical practice.
What are the main challenges companies face when attempting to comply with 21 CFR PART 11? How can an LMS (Learning Management System) help your company streamline compliance? Our quality experts explain further in this article.
What is 21 CFR Part 11?
21 CFR Part 11, issued by the FDA (Food and Drug Administration), is a set of standards for electronic documents, electronic signatures and computer systems in the life sciences sector. These requirements ensure that all electronic documents are secure, reliable, and comparable to physical documents (paper equivalence). This means that all systems must guarantee the highest level of authentication and data security for all electronic documents, signatures, and systems.
Part 11 addresses the challenges of balancing innovation, reliability and data integrity in the life sciences, CROs, medical devices and manufacturing industries.
Challenges in achieving 21 CFR Part 11 Compliance
Over the period 2016-2020, the FDA Dashboard shows the most frequent issues of noncompliance for companies attempting to meet 21 CFR Part 11 compliance:
- Data integrity risks: The most common noncompliance issues concern inadequate data storage procedures. Thus, FDA inspections identify the need to have tamper-proof electronic records and comprehensive audit trails, ensuring all electronic records are accurate and secure.
- System validation: The FDA’s inspection data also identified inadequate validation processes, leading to delays in clinical trial approvals and increased inspections by regulatory authorities.
- Training and documentation: FDA Inspections found weaknesses in training documentation and a lack of understanding of compliance protocols by employees.
- Audit readiness: Maintaining audit-ready systems is a challenge for companies. Often, audit trails are inadequate with fragmented documentation.
- User authentication: The final common issue highlighted is inadequate electronic signature protocols. A weakness that increases the risk of data falsification and unauthorized access.
These 5 challenges are most frequently identified during FDA inspections. How can they be effectively addressed? With validated Learning Management Systems (LMS), pharmaceutical and biotech companies can strengthen their compliance with regulatory requirements and improve their readiness for FDA inspections.
How Dokeos LMS Ensures 21 CFR Part 11 Compliance
Dokeos LMS is a fully validated, GxP-compliant platform designed to meet the needs of pharmaceutical and biotech companies. Our platform integrates essential compliance tools with user-friendly features to ensure data integrity and achieve operational excellence.
Simplifying compliance with Dokeos
Dokeos LMS simplifies 21 CFR Part 11 compliance with secure, tamper-proof audit trails, electronic signature validation, automated compliance tracking, and Cloud GxP compliance. Only authorized personnel – quality managers, HR, and training managers – can access or modify individual training files and other records. Every user action is traceable, with time-stamped entries guaranteeing complete traceability and audit-readiness.
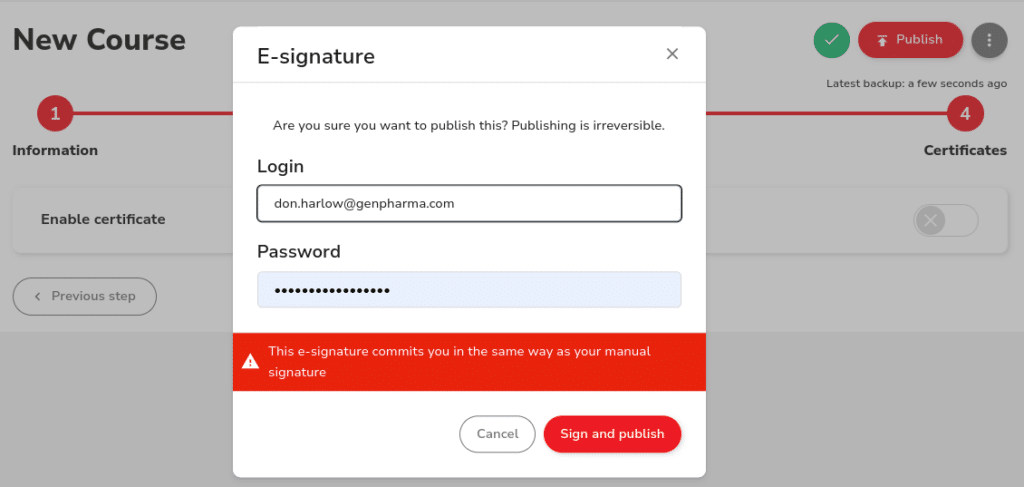
Integration for streamlined workflows
Dokeos LMS integrates seamlessly with your HR and Quality Management Systems (QMS), consolidating training, employee records, and compliance documentation into a single centralized management center.
Why Choose Dokeos LMS for 21 CFR Part 11 Compliance ?
Dokeos LMS is more than just a compliance tool; it’s a strategic partner that simplifies compliance, improves operational efficiency, and stimulates innovation. We offer a validated LMS that integrates with your quality assurance process through audit trails, SOP management, versioning, training records, electronic signature management, and corrective and preventive actions. What’s more, our IT system validation enables you to guarantee paper equivalence in a cloud-based solution.
We address security risks by providing threat modeling and security design reviews. We provide quarterly code assessments and ensure that employees have ticked the “read and understood” box on all SOPs of their individual learning paths. In addition, our advanced audit testing functionality captures and records all user activity, providing time-stamped records and ensuring full traceability during inspections.
Our partner, GxP-Training, offers an Introduction to FDA 21 CFR PART 11 online course that provides learners with a dated, traceable, and downloadable course that can be validated online.
Discover how Dokeos LMS can streamline your compliance efforts by enhancing your training efficiency.
Start your free trial or schedule a personalized demo today.
FAQs
What is FDA CFR Part 11 compliance?
CFR Part 11 is a set of requirements published by the FDA that ensure all electronic records and signatures are reliable, trustworthy, and comparable to physical paper records.
What is FDA 21 CFR Part 11 compliance checklist?
Find out how Dokeos perfectly meets the 21 CFR Part 11 compliance checklist. Our LMS is designed to meet the latest GxP compliance standards.
What are the penalties for non-compliance with 21 CFR Part 11?
Penalties for non-compliance can range from fines and product recalls to loss of regulatory approval and criminal charges. The FDA provides a list of notices of noncompliance and penalties.















