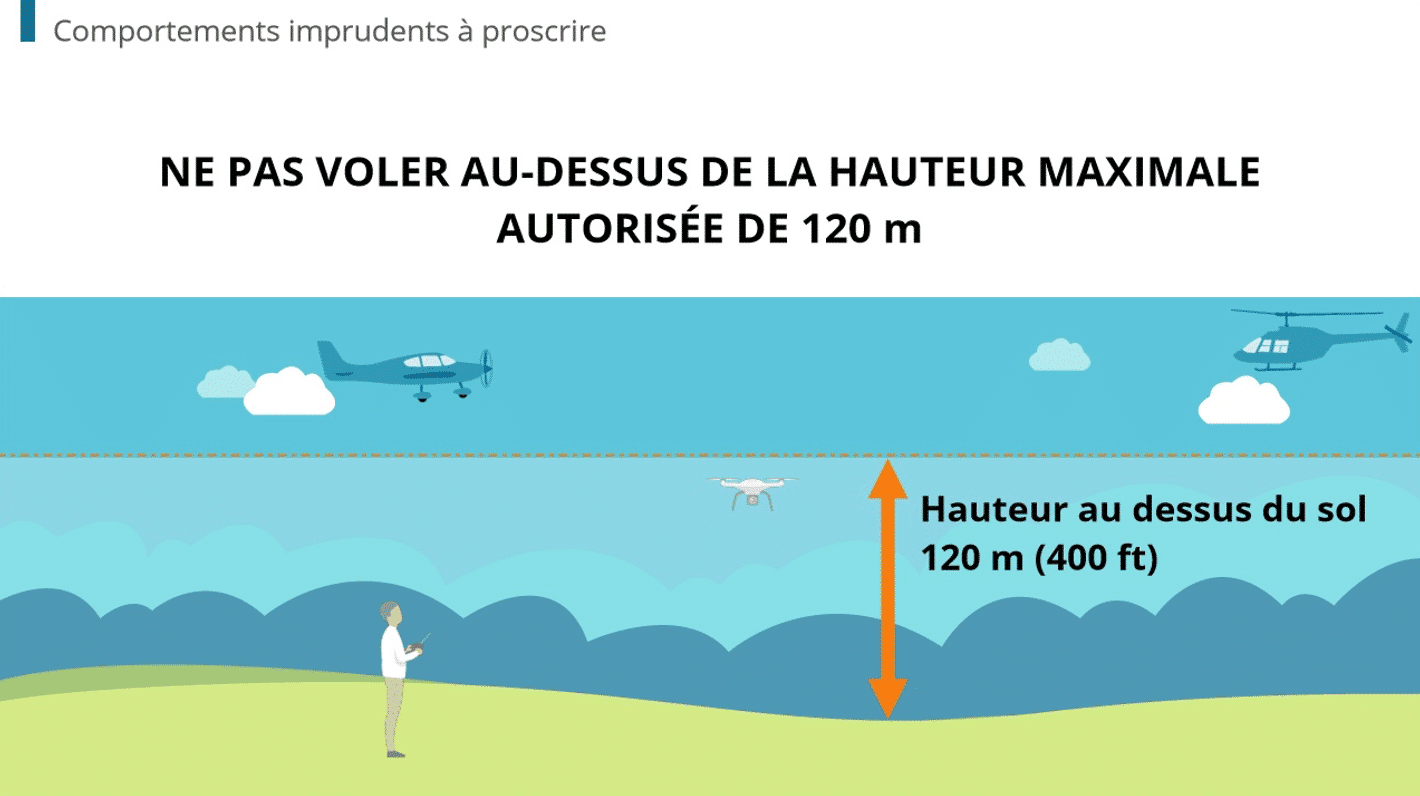
FDA proposes that drug manufacturing companies should calculate four KPIs to prevent shortages and uphold QA standards. In this article, I’ll explore 7 ways your can use Dokeos LMS to collect, manage, and aggregate quality control metrics.
How Dokeos LMS Helps Tracking Of FDA’s Quality Control Metrics
In 2015, the FDA released their “Request for Quality Metrics – Guidance to Industry” report, which outlines four KPIs for the drug manufacturing industry. These include: low acceptance, invalidated out of specification, product quality review on time, and product quality compliant rates. This document is intended as a guide for now. However, drug manufacturing companies should already be tracking their KPIs to improve work practices and comply with industry standards.
Fortunately, there are LMS platforms that can help you stay in compliance and track quality control metrics. One of the most notable examples is Dokeos LMS, which specializes in corporate compliance training. Here are just a few of the features and functionalities of the Dokeos LMS that can help pharmaceutical companies monitor performance indicators and ensure Quality Assurance.
1. FDA And EMA Compliant Learning Management System
Dokeos LMS gives you the power to incorporate FDA and EMA requirements into the Compliant Learning Management System. It’s also compliant with FDA 21 CFR PART11. As such, you can integrate electronic signatures, create different versions of reviews, and capture compliance data. There is no need to invest in additional tools in order to track performance and quality metrics. This makes Dokeos LMS the ideal Learning Management System for pharmaceutical manufacturers, laboratories, and other life science industries.
2. Data Protection
Sensitive data requires advanced encryption and security protocols. This is particularly true in pharmaceutical manufacturing industries where there may be security audits by governmental agencies. Dokeos LMS provides extensive security protection measures to ensure that your LMS metrics and data remain well guarded. Thus, you don’t have to worry about your KPI reports being compromised during a security breach.
3. Validate Standard Operating Procedures (SOPs) With Dokeos EVALUATION
Dokeos LMS allows you to validate Standard Operating Procedures (SOPs) and employee skills. As a result, you have access to online training files for every member of your organization in the event of an audit. You’re able to provide proof that employees are up-to-date with compliance and certifications. This is further aided by the electronic signature feature that Dokeos LMS provides.
4. Clinical Analysis To Improve And Monitor Employee Performance
The DOKEOS Evaluation tool allows you to create both qualitative and quantitative assessments. Therefore, you can monitor employee performance and periodically check their professional knowledge base. The Dokeos LMS even features sub-questions that enable you to test procedural experience and clinical reasoning skills.

5. Certification Support
There are some compliance issues that require certifications. Dokeos LMS gives you the ability to issue certificates based on specific criteria. As a result, you can ensure that every employee is up to code and has the personalized online training resources they need. This helps your organization authenticate and validate individual competencies and skill sets.
6. Monitor Online Training Initiatives And Metrics With Dokeos MANAGER
DOKEOS Manager has a broad range of features, from curriculum organization to online training personalization. However, its standout function is the ability to track quality and performance metrics. You have the ability to monitor overall results, participation rates, and assessment scores. It even boasts visual reports so that you can track patterns and trends in the LMS.
7. Hire Employees Who Have The Necessary Skill Sets And Experience
One of the most effective ways to meet FDA requirements is to recruit employees who already have the necessary skills and experience. For example, those who are familiar with compliance protocols and FDA or EMA regulations. Dokeos LMS offers a human resources tool that allows you to identify and vet top candidates. As such, you find the most qualified person for the job based on employee competencies.
Other Stand Out Dokeos LMS Features That Reduce Risk And Improve Online Training ROI
FDA and EMA compliance are a major factor in the decision-making process. However, there are other considerations when choosing the best LMS for your organization. Here are a few more stand out features that Dokeos LMS offers.
a. App Integration
Dokeos LMS provides an entire suite of software to maximize online training effectiveness, including Dokeos Manager, Author, and Live. You can even integrate serious games into your online training strategy to build real-world experience and improve proficiency. The Dokeos LMS platform is also SCORM compliant and supports social learning activities. For example, online instructors and employees can interact via online forums, social media sites, and online chats, or access online training course calendars and notifications to stay in the loop. This facilitates a thriving compliance online training culture that is fully transparent and collaborative.
b. Responsive Design
Employees, developers, and administrators require remote access to the online training system. Dokeos LMS is a responsive design platform, which means that you’re able to login to the tool using any device. A side benefit of this is not having to update the software on a regular basis, not to mention that the set up process is simple and straightforward. The responsive design feature is also useful during audits due to the fact that you have all the compliance data on hand.
c. Asset Repurposing
Dokeos LMS offers you the ability to incorporate your existing online training content. As such, you can repurpose current compliance activities and resources and update them to reflect current rules and regulations. This cuts online training costs and allows you to develop and deploy crucial QA training initiatives as quickly as possible.
The FDA’s focus on metrics, performance, and quality may lead to fewer drug shortages and reduced consumer risk. However, you need an LMS that can help you develop, deploy, and monitor compliance and competencies to meet the requirements. Thankfully, Dokeos LMS does offer a free 60-day trial so that you can test out the features. This gives you the opportunity to see if it’s a good fit for your compliance and QA online training needs.